Coho salmon in the Pacific Northwest have been dying in record numbers for the past few years due to what researchers call “Urban runoff mortality syndrome” (URMS). (Videos of adult male coho salmon with symptoms of URMS available here and here). In the Science publication a team of researchers from Washington, California, and Toronto, Canada have characterized the roadway run-off and run-off water in several sites in Seattle, one site in Los Angeles, and one site in the San Francisco Bay area. Their analysis has uncovered toxic levels of the compound N-(1,3-dimethylbutyl)-N'-phenyl-p-phenylenediamine) (6PPD), a common antioxidant in tire rubber. Considering 3.1 billion tires are produced worldwide annually this contributes to an estimated average of 0.81 kg/capita annual emission of tire rubber particles containing this antioxidant. The complete article is included below.
ABSTRACT:
“In U.S. Pacific Northwest coho salmon (Oncorhynchus kisutch), stormwater exposure annually causes unexplained acute mortality when adult salmon migrate to urban creeks to reproduce. By investigating this phenomenon, we identified a highly toxic quinone transformation product of N-(1,3-dimethylbutyl)-N'-phenyl-p-phenylenediamine) (6PPD), a globally ubiquitous tire rubber antioxidant. Retrospective analysis of representative roadway runoff and stormwater-impacted creeks of the U.S. West Coast indicated widespread occurrence of 6PPD-quinone (<0.3-19 μg/L) at toxic concentrations (LC50 of 0.8 ± 0.16 μg/L). These results reveal unanticipated risks of 6PPD antioxidants to an aquatic species and imply toxicological relevance for dissipated tire rubber residues.”
Figure 4 Environmental relevance of 6PPD-quinone.
(A) Using retrospective UPLC-HRMS analysis of archived sample extracts, 6PPD-quinone was quantified in roadway runoff and runoff-impacted receiving waters. Each symbol corresponds to duplicate or triplicate samples, boxes represent first and third quartiles. For comparison, the 0.8 μg/L LC50 value for juvenile coho salmon and detected 6PPD-quinone levels in 250 and 1000 mg/L TWP leachate are included. (B) Predicted ranges of potential 6PPD-quinone mass formation in passenger cars (e.g., 4 tires, ~36 kg tire rubber mass) and heavy trucks, (e.g., 18 tires, ~900 kg of tire rubber) (represented in orange) and measured 6PPD-quinone concentrations in affected environmental compartments (represented in blue, with experimental data italicized). Predicted ranges reflect calculations applying 0.4-2% 6PPD per total vehicle tire rubber mass followed by various yield scenarios (1-75% ultimate yields) for 6PPD reaction with ground-level ozone to form 6PPD-quinone.
AUTHORS:
Zhenyu Tian 1,2, Haoqi Zhao 3, Katherine T. Peter 1,2, Melissa Gonzalez 1,2, Jill Wetzel 4, Christopher Wu 1,2, Ximin Hu 3, Jasmine Prat 4, Emma Mudrock 4, Rachel Hettinger 1,2, Allan E. Cortina 1,2, Rajshree Ghosh Biswas 5, Flávio Vinicius Crizóstomo Kock 5, Ronald Soong 5, Amy Jenne 5, Bowen Du 6, Fan Hou 3, Huan He 3, Rachel Lundeen 1,2, Alicia Gilbreath 7, Rebecca Sutton 7, Nathaniel L. Scholz 8, Jay W. Davis 9, Michael C. Dodd 3, Andre Simpson 5, Jenifer K. McIntyre 4, Edward P. Kolodziej 1,2,3,*
Center for Urban Waters, Tacoma, WA 98421, USA.
Interdisciplinary Arts and Sciences, University of Washington Tacoma, Tacoma, WA 98421, USA.
Department of Civil and Environmental Engineering, University of Washington, Seattle, WA 98195, USA.
School of the Environment, Washington State University, Puyallup, WA 98371, USA.
Department of Chemistry, University of Toronto, Scarborough Campus, 1265 Military Trail, Toronto, ON M1C1A4, Canada.
Southern California Coastal Water Research Project, Costa Mesa, CA 92626, USA.
San Francisco Estuary Institute, 4911 Central Avenue, Richmond, CA 94804, USA.
Environmental and Fisheries Sciences Division, Northwest Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Administration, Seattle, WA 98112, USA.
United States Fish and Wildlife Service, Washington Fish and Wildlife Office, Lacey, WA 98503, USA.
*Corresponding author. Email: koloj@uw.edu
Humans discharge tens of thousands of chemicals and related transformation products to water (1), most of which remain unidentified and lack rigorous toxicity information (2). Efforts to identify and mitigate high risk chemical toxicants are typically reactionary, occur long after their use becomes habitual (3), and are frequently stymied by mixture complexity. Societal management of inadvertent, yet widespread, chemical pollution is therefore costly, challenging, and often ineffective.
The pervasive biological degradation of contaminated waters near urban areas (i.e., “urban stream syndrome”) (4) is exemplified by an acute mortality phenomenon affecting Pacific Northwest coho salmon (Oncorhynchus kisutch) for decades (5–9). “Urban runoff mortality syndrome” (URMS) occurs annually among adult coho salmon returning to spawn in freshwaters where concurrent stormwater exposure causes rapid mortality. In the most urbanized watersheds with extensive impervious surfaces, 40-90% of returning salmon may die before spawning (9). This mortality threatens salmonid species conservation across ~40% of Puget Sound land area despite costly societal investments in physical habitat restoration that may have inadvertently created ecological traps due to episodic toxic water pollution (9). Although URMS has been linked to degraded water quality, urbanization, and high traffic intensity (9), one or more causal toxicants have remained unidentified. Spurred by these compelling observations and mindful of the many other insidious sublethal stormwater impacts, we have worked to characterize URMS water quality (10, 11).
Previously, we reported that URMS-associated waters had similar chemical compositions relative to roadway runoff and tire tread wear particle (TWP) leachates, providing an opening clue in our toxicant search (10). Here, we applied hybrid toxicity identification evaluation and effect-directed analysis to screen TWP leachate for its potential to induce mortality (a phenotypic anchor) in juvenile coho salmon as an experimental proxy for adult coho (6). Using structural identification via ultrahigh performance liquid chromatography-high resolution tandem mass spectrometry (UPLC-HRMS/MS) and nuclear magnetic resonance (NMR), we discovered that an antioxidant-derived chemical was the primary causal toxicant. Retrospective analysis of runoff and receiving waters indicated that detected environmental concentrations of this toxicant often exceeded acute mortality thresholds for coho during URMS events in the field and across the U.S. West Coast.
Aqueous TWP leachate stock (1000 mg/L) was generated from an equal-weight mix of tread particles (0.2 ± 0.3 mm2 average surface area) (fig. S1) from nine used and new tires (table S1). TWP leachate (250 mg/L positive controls) was acutely and rapidly (~2-6 hours) lethal to juvenile coho (24 hours exposures, 98.5% mortality, n = 135 fish from 27 exposures, see Data S1), even after heating (80°C, 72 hours; 100% mortality, n = 10 fish from two exposures), indicating stability during handling. Behavioral symptomology (circling, surface gaping, equilibrium loss) (fig. S2 and movie S1) of TWP leachate exposures mirrored laboratory and field observations of symptomatic coho (5, 6). No mortality occurred in negative controls, including solvent- and process-matched method blanks subjected to identical separations (0 of 80 fish, 16 exposures) or exposure water blanks (0 of 45 fish, 9 exposures).
Mixture complexity (measured here as number of UPLC-HRMS electrospray ionization (ESI+) chemical features) was a significant barrier to causal toxicant identification, as 250 mg/L TWP leachate typically contained >2000 ESI+ detections. Our fractionation studies, optimized over 2+ years via iterative exploration of toxicant chemical properties, focused on reducing these detection numbers to attain a simple, yet toxic, fraction amenable to individual compound identifications. Throughout this fractionation procedure, observed toxicity remained confined to one narrow fraction, consistent with a single compound or a small, structurally related family of causal toxicants. In initial studies, TWP leachate toxicity was unaffected by silica sand filtration, cation and anion exchange, and ethylenediaminetetraacetic acid (EDTA, 114 μM) addition (12), indicating that toxicant(s) were not particle-associated, strongly ionic, or metals, respectively, and validating prior studies that eliminated candidate pollutants (13, 14) as primary causal toxicants.
Mixture complexity was reduced using cation exchange, two polarity-based separations (XAD-2 resin and silica gel), and reverse phase high-performance liquid chromatography (HPLC) on a semi-preparative C18 column (250×4.2 mm ID, 5 μm particle size). After C18-HPLC generated ten fractions, only C18-F6 (10-11 min) was toxic; it contained ~225 ESI+ and ~70 ESI- features (Fig. 1). Having removed ~90% of features, we began to prioritize and identify candidate toxicants by abundance (peak area), followed by fish exposures with commercial standards at 5-fold higher concentrations (mixtures at 1-25 μg/L) than those estimated in C18-F6. We identified eleven plasticizers, antioxidants, emulsifiers, and various transformation products, including some well-known environmental contaminants (e.g., tris(2-butoxyethyl) phosphate) and some that are rarely reported [e.g., di(propylene glycol) dibenzoate, 2-(1-phenylethyl)phenol] (table S2). We also detected several bioactive, structurally related phenolic antioxidants and their transformation products (2,6-di-t-butyl-4-hydroxy-4-methyl-2,5-cyclohexadienone, 3,5-di-t-butyl-4-hydroxybenzaldehyde, 7,9-di-tert-butyl-1-oxaspiro[4,5]deca-6,9-diene-2,8-dione) (15). However, over many rounds of identification and subsequent exposure to juvenile coho, none of these identified chemical exposures reproduced URMS symptoms or induced mortality. As these identifications employed exhaustive environmental scientific literature searches (10, 16, 17), we suspected a previously unreported toxicant.
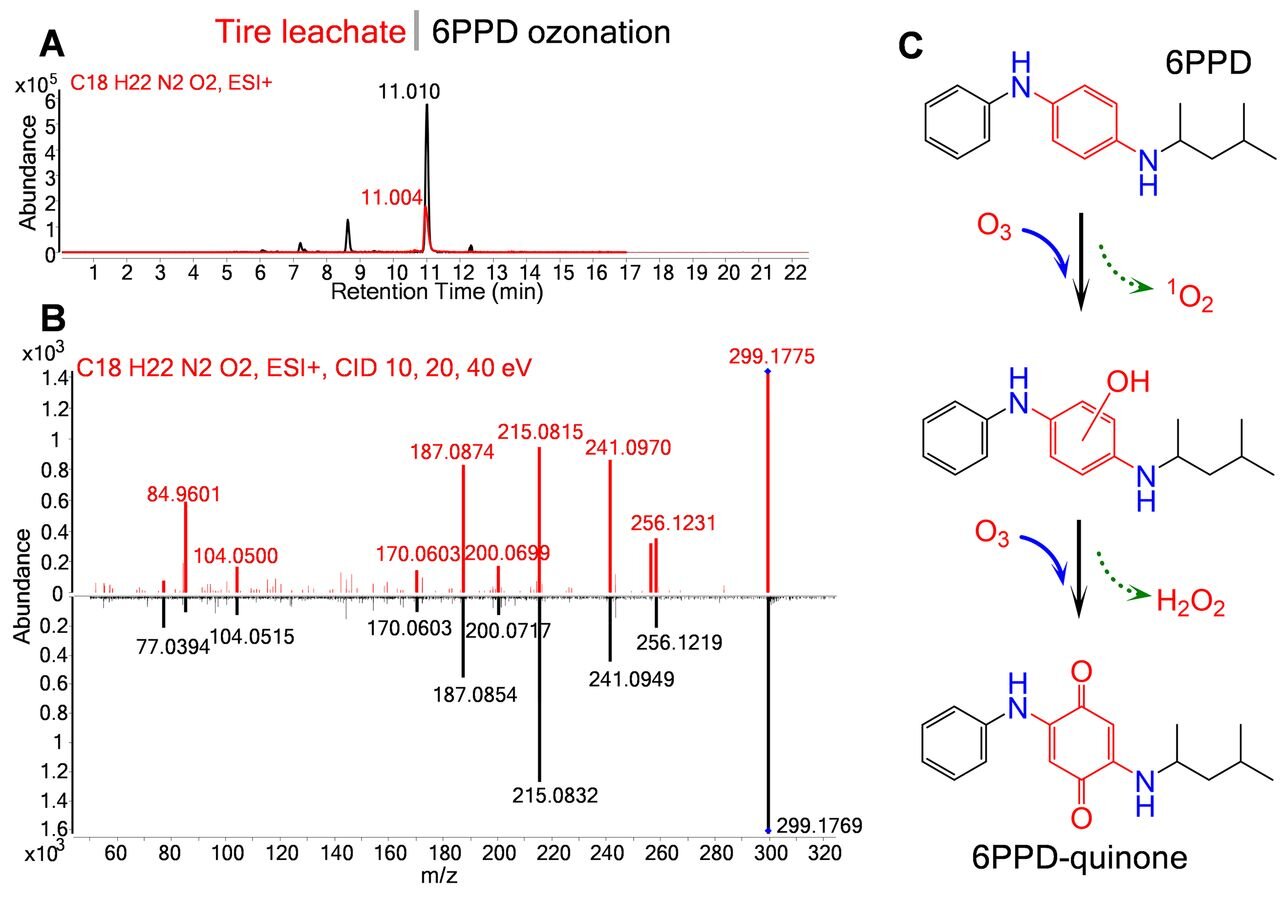
To sharpen our search, we employed multi-dimensional semi-preparative HPLC using two additional structurally distinct column phases (pentafluorophenyl (PFP) and phenyl). Parallel fractionations (18) (same column dimensions, mobile phase, and gradient as for C18-HPLC) of the toxic silica gel fraction generated toxic fractions of PFP-F6 (10-11 min; ~204 ESI+, 60 ESI– features) and phenyl-F4 (8-9 min; ~237 ESI+, 75 ESI– features); all other fractions were non-toxic. Notably, across these separations (C18, PFP, phenyl), only 4 ESI+ and 3 ESI- HRMS features co-occurred in all three toxic fractions (fig. S3). Of these, one unknown compound (m/z 299.1752, C18H22N2O2, RT 11.0 min on analytical UPLC-HRMS) dominated the detected peak area (10-fold higher intensity in both ESI+ and ESI–). To further resolve candidate toxicants for synthetic efforts, we converted the three-dimensional chromatography workflow from parallel to serial through sequential C18, PFP, and phenyl columns (C18-F6 to PFP-F6 to phenyl-F4; with solvent removal by centrifugal evaporation and toxicity confirmation between separations). The purified final fraction was chemically simple (4 ESI+, 3 ESI– detections), highly lethal (100% mortality in 4 hours; n = 15 coho, 3 exposures), and was again dominated by C18H22N2O2. Drying this fraction yielded a pink-magenta precipitate (Fig. 1).
Figure 1 Tire rubber leachate fractionation scheme.
As a metric of mixture complexity and separation efficiency, the numbers above gray bars represent unique chemical features detected in solid-phase extracted fish exposure water (1 L) and subsequent fractions by UPLC-HRMS. Blue colors represent non-lethal fractions; red colors represent lethal fractions. All fractionation steps and exposures were replicated at least twice; positive and negative controls were included throughout fractionations. The inset photo depicts purified product (~700 μg from 30 L of TWP leachate) in the final lethal fraction. TWP, tire tread wear particles; CEX, cation exchange; EA, ethyl acetate; EtOH, ethanol; H2O, water; Hex, hexane; DCM, dichloromethane; RT, retention time.
Published characterizations of crumb rubber (16) and receiving waters (10, 17) did not mention C18H22N2O2. UPLC-HRMS/MS spectra indicated C4H10 and C6H12 alkyl losses (M-58 and M-84 fragments) (Fig. 2B), but MS3 and MS4 fragmentation yielded no additional structural insights (fig. S4). Additionally, in silico fragmentation (MetFrag, CSI:FingerID) of C18H22N2O2 compounds in PubChem and ChemSpider (15,624 and 17,105 structures, respectively) failed to match observed fragments. Thus, to the best of our knowledge, C18H22N2O2 was not described in environmental literature or databases and posed a “true unknown” identification problem (19). We now assumed a transformation product; industrial manufacturing (e.g., high heat/pressure, catalysis) and diverse reactions in environmental systems generate many undocumented transformation products, most of which lack commercial standards.
Figure 2 6PPD-quinone identification and a proposed formation pathway.
(A) Extracted ion chromatograms of 6PPD-quinone from UPLC-HRMS (ESI+); red data represents the final fraction from TWP leachate, while black data represents the purified 6PPD ozonation mixture. (B) Observed MS/MS fragmentation (integrated from 10, 20, 40 eV) of 6PPD-quinone in the final toxic fraction from TWP leachate (red spectra) and 6PPD ozonation (black spectra). (C) One proposed reaction pathway from 6PPD to 6PPD-quinone (see fig. S13 for alternate proposed formation pathways). Red highlights detail key changes in the diphenylamine structure during ozonation.
Our breakthrough came by assuming that abiotic environmental transformations commonly modify active functional groups by preferentially altering the numbers of hydrogen and oxygen atoms relative to carbon and nitrogen. By searching a recent EPA crumb rubber report (16) for related formulas (i.e., C18H0-xN2-4O0-y), several characteristics of the C18H24N2 anti-ozonant “6PPD” [N-(1,3-dimethylbutyl)-N'-phenyl-p-phenylenediamine] matched necessary attributes. First, 6PPD is globally ubiquitous (0.4-2% by mass) in passenger and commercial vehicle tire formulations (20), indicating sufficient production to explain mortality observations within large and geographically distinct receiving water volumes. 6PPD was present in TWP leachate but was completely removed during fractionation by cation exchange. 6PPD crystals are purple, similar to the pink-magenta precipitate obtained post-fractionation. Most compellingly, neutral losses in 6PPD GC-MS spectra matched the C18H22N2O2 GC-HRMS spectra (fig. S5) and the predicted logKow of 6PPD (5.6) was close to that for C18H22N2O2 (5-5.5) (11). Finally, literature detailing the industrial chemistry of 6PPD reactions with ozone (7 day, 500 ppbv) described a C18H22N2O2 product (21), leading us to hypothesize that 6PPD was the likely pro-toxicant (Fig. 2C).
We tested this hypothesis with gas-phase ozonation (500 ppbv O3) of industrial grade 6PPD (96% purity) (21). A C18H22N2O2 product formed; UPLC-HRMS analysis demonstrated exact matches of retention time (11.0 min) and MS/MS spectra between this synthetic C18H22N2O2 and the TWP leachate fractionation-derived C18H22N2O2 (Fig. 2, A and B). When purified, the ozone-synthesized C18H22N2O2 formed a reddish-purple precipitate. 1D 1H NMR structural analysis confirmed identical TWP leachate-derived and ozone-synthesized C18H22N2O2 structures (figs. S6 to S7). Notably, 2D NMR spectra and related simulations revealed isolated tertiary carbons and carbonyl groups (figs. S8 to S12), clearly indicating a quinone structure for C18H22N2O2 rather than the dinitrone structure reported in the past 40 years of literature describing 6PPD ozonation products (21). Therefore, the C18H22N2O2 candidate toxicant was unequivocally “6PPD-quinone” (2-anilino-5-[(4-methylpentan-2-yl)amino]cyclohexa-2,5-diene-1,4-dione). Consistent with environmental 6PPD ozonation, reported 6PPD ozonation products C18H22N2O (formula-matched) and 4-nitrosodiphenylamine (C12H10N2O, standard-confirmed) (21) also were detected in ozonation mixtures and non-toxic TWP leachate fractions.
Exposures to ozone-synthesized and tire leachate-derived 6PPD-quinone (~20 μg/L nominal concentrations) both induced rapid (<5 hours, with initial symptoms evident within 90 min) mortality (n = 15 fish, 3 exposures) (fig. S2 and movie S2) which matched the 2-6 hours mortality observed for positive controls. Behavioral symptomology in response to synthetic 6PPD-quinone exposures matched that from field observations, roadway runoff, bulk TWP leachate and final toxic TWP fraction exposures, confirming the phenotypic anchor (5–9). Using synthetic 6PPD-quinone (purity ~98%), controlled dosing experiments (10 concentrations, n = 160 fish in two independent exposures) were performed. 6PPD-quinone was highly toxic (LC50 0.79 ± 0.16 μg/L) to juvenile coho salmon (Fig. 3B). Estimates of LC50 via controlled exposures closely matched estimates derived from bulk roadway runoff and TWP leachate exposures (LC50 0.82 ± 0.27 μg/L), indicating the primary contribution of 6PPD-quinone to observed mixture toxicity (Fig. 3A). Direct comparisons with 6PPD were performed (LC50 250 ± 60 μg/L via nominal concentrations) (fig. S14), but confident assessment of 6PPD toxicity was precluded by its poor solubility, high instability, and formation of products during exposure.
To assess environmental relevance, we used UPLC-HRMS to retrospectively quantify 6PPD-quinone in archived extracts from roadway runoff and receiving water sampling (fig. S15 and table S4) (10). In Seattle-region roadway runoff (n = 16/16), 0.8-19 μg/L 6PPD-quinone was detected (Fig. 4A). During seven storm events in three Seattle-region watersheds highly impacted by URMS, 6PPD-quinone occurred at <0.3-3.2 μg/L (n = 6/7 discrete storm events; n = 6/21 samples when including samples collected across the full hydrograph). These samples included three storms with documented URMS mortality in adult coho salmon: 6PPD-quinone was not detected in pre- and post-storm samples, but concentrations were near or above LC50 values during storms. We also detected 6PPD-quinone in Los Angeles region roadway runoff (n = 2/2, 4.1-6.1 μg/L) and San Francisco region creeks impacted by urban runoff (n = 4/10, 1.0-3.5 μg/L).
Figure 4 Environmental relevance of 6PPD-quinone.
(A) Using retrospective UPLC-HRMS analysis of archived sample extracts, 6PPD-quinone was quantified in roadway runoff and runoff-impacted receiving waters. Each symbol corresponds to duplicate or triplicate samples, boxes represent first and third quartiles. For comparison, the 0.8 μg/L LC50 value for juvenile coho salmon and detected 6PPD-quinone levels in 250 and 1000 mg/L TWP leachate are included. (B) Predicted ranges of potential 6PPD-quinone mass formation in passenger cars (e.g., 4 tires, ~36 kg tire rubber mass) and heavy trucks, (e.g., 18 tires, ~900 kg of tire rubber) (represented in orange) and measured 6PPD-quinone concentrations in affected environmental compartments (represented in blue, with experimental data italicized). Predicted ranges reflect calculations applying 0.4-2% 6PPD per total vehicle tire rubber mass followed by various yield scenarios (1-75% ultimate yields) for 6PPD reaction with ground-level ozone to form 6PPD-quinone.
These data implicate 6PPD-quinone as the primary causal toxicant for decades of stormwater-linked coho salmon acute mortality observations. While minor contributions from other constituents in these complex mixtures are possible, 6PPD-quinone was both necessary (i.e., consistently present in and absent from toxic and non-toxic fractions, respectively) and, when purified or synthesized as a pure chemical exposure, sufficient to produce URMS at environmental concentrations. Over the product life cycle, antioxidants (e.g., PPDs, TMQs, phenolics) are designed to diffuse to tire rubber surfaces, rapidly scavenge ground-level atmospheric ozone and other reactive oxidant species, and form protective films to prevent ozone-mediated oxidation of structurally significant rubber elastomers (21, 22). Accordingly, all 6PPD added to tire rubbers is designed to react, intentionally forming 6PPD-quinone and related transformation products that are subsequently transported through the environment. This anti-ozonant application of 6PPD inadvertently, yet drastically, increases roadway runoff toxicity and environmental risk by forming the more toxic and mobile 6PPD-quinone transformation product. Based on the ubiquitous use and substantial mass fraction (0.4-2%) of 6PPD in tire rubbers and the representative detections across the U.S. West Coast (table S4), which include many detections near or above LC50 values, we believe that 6PPD-quinone may be present broadly in peri-urban stormwater and roadway runoff at toxicologically relevant concentrations for sensitive species, such as coho salmon.
Globally, ~3.1 billion tires are produced annually for our >1.4 billion vehicles, resulting in an average 0.81 kg/capita annual emission of tire rubber particles (23). TWPs are one of the most significant microplastics sources to freshwaters (24); 2-45% of total tire particle loads enter receiving waters (25, 26) and freshwater sediment contains up to 5800 mg/kg TWP (23, 24, 27). Supporting recent concerns about microplastics (24, 28), 6PPD-quinone provides a compelling mechanistic link between environmental microplastic pollution and associated chemical toxicity risk. While numerous uncertainties exist regarding the occurrence, fate, and transport of 6PPD-quinone, these data indicate that aqueous and sediment environmental TWP residues can be toxicologically relevant and existing TWP loading, leaching, and toxicity assessments in environmental systems are clearly incomplete (25). Tire rubber disposal also represents a major global materials problem and potential potent source of 6PPD-quinone and other tire-derived transformation products. In particular, scrap tires re-purposed as crumb rubber in artificial turf fields (17) suggest both human and ecological exposures to these chemicals. Accordingly, the human health effects of such exposures merit evaluation.
Environmental discharge of 6PPD-quinone is particularly relevant for the many receiving waters proximate to busy roadways (Fig. 4B). It is unlikely that coho salmon are uniquely sensitive, and the toxicology of 6PPD transformation products in other aquatic species should be assessed. For example, used tires were more toxic to rainbow trout (4-fold lower 96-hours LC50) relative to new tires (29), an observation consistent with adverse outcomes mediated by transformation products. If management of 6PPD-quinone discharges is needed to protect coho salmon or other aquatic organisms, adaptive regulatory and treatment strategies (17, 30, 31) along with source control and “green chemistry” substitutions (i.e., identifying demonstrably non-toxic and environmentally benign replacement antioxidants (22, 32)) can be considered. More broadly, we recommend more careful toxicological assessment for transformation products of all high production volume commercial chemicals subject to pervasive environmental discharge.
Supplementary data are included here.